Browse Careers
Discover
what is possible
at Duke Health
At Duke Health, every team member plays a role in advancing health – no matter the position. Explore open roles and learn how you can make an impact in health care.
Browse All Jobs
Administrative and Support Services
Help to support healthcare functions and keep facilities running smoothly as a dedicated administrative and support services professional.

Advanced Practice Providers
Bring your passion for caring and discovery to Duke Health as a critical member of the care team.

Allied Health
Join the talented allied health professionals that are a key part of the medical team supporting patient care.

Corporate
Apply your expertise to positively impact patient care through a range of positions including human resources, accounting and finance, performance services, and marketing and communications.

Information Technology
Find your place with Duke Health in one of our specialized divisions as an information technology professional.

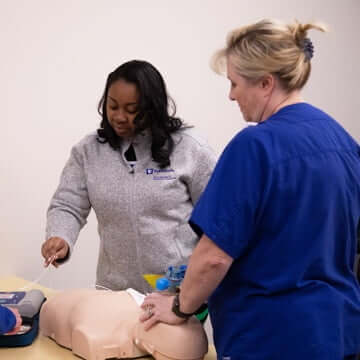
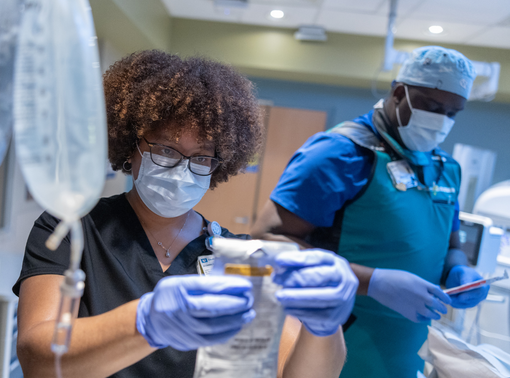
Nursing and Clinical Support
Make a difference in the lives of patients and their families as a nursing and clinical support team member.

Physicians
Join a team making historic breakthroughs that are positively impacting patient care and helping us to carry out our mission of advancing health together.

Revenue Management
Assist patients and their families with registration, coding, and billing for our care facilities.
Careers at Duke Health
Team members at Duke Health are a big part of how we are able to deliver compassionate care to patients. Together, we partner to create an inclusive environment promoting learning and the breakthroughs we are known for across the country. Hear from a few team members about why they chose Duke Health.
Perks and Benefits
We are here to support you with perks and benefits to keep you happy and healthy in both your career and your life.
Locations Across the Triangle
With hospitals in Raleigh and Durham and more than 140 specialty and primary care clinics in and around the Triangle region, there are many options to join Duke Health in the city and the suburbs.




Core Duke Health Divisions
Awards and Recognition
Duke Health is consistently recognized as a top employer for our dedication to the team members and the patients we serve.

On Forbes list of Best Large Employers, we are the #1 healthcare recipient in North Carolina for 2023.

Our hospitals are consistently recognized as LGBTQ Healthcare Equality Leaders by Healthcare Equality Index.

We are among about 9 percent of U.S. hospitals that hold the American Nurses Credentialing Center’s Magnet Recognition honor.
CHIME Digital Health Most Wired recipient since 2018, honoring our ability to elevate the health and care of communities around the world.

We are proud to be named as the #1 healthcare system in North Carolina on Fortune’s America’s Most Innovative Companies 2023 list.
Jobs For You
As you search for opportunities that fit your skills, any jobs you view will appear here.
You haven’t saved any jobs yet, but when you find an opportunity that seems right for you, bookmark it so you can return to it easily.